|
Dr. Gordon Walker discusses Oxidation Reduction Potential (ORP) as process parameter for the wine industry. He covers the theory and importance of redox reactions in determining the growth of microorganisms. He presents information from other industries where ORP has been used effectively to control their fermentations. He discusses the benefits of using ORP/Redox control in wine fermentations - leading to less "reductive" characters and faster/more robust fermentations. Data from multiple harvests and facilities is presented, showing how ORP corresponds to yeast metabolism in wine ferments. He concludes that redox potential/ORP can be used effectively in the wine industry to increase process control and security. Email Dr. Gordon A. Walker: [email protected] if you have further questions.
0 Comments
Dr. Gordon A. Walker Discusses Redox Potential As a Process Parameter for Wine MakersAs part of the UC Davis Flavor 101 Lecture Series, Gordon Walker presents his research on oxidation reduction potential during fermentation as part of the January 9th event titled "Microbial Terroir - Science and Practice".
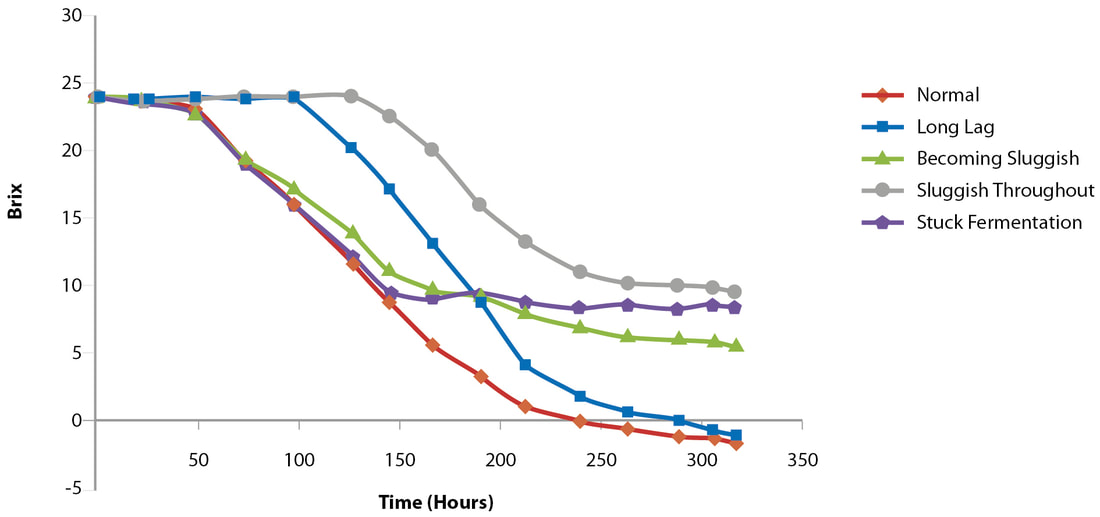 Just in time for harvest! When considering fermentations, the most important considerations are choice of strain as how it relates to wine style, nitrogen and vitamin requirements, temperature and ethanol tolerance, microbial competitiveness and over all fermentation capacity. We are fortunate to live in a world with a plethora of solid commercial options for strains, nutrition products, and advice for avoiding problem fermentations. Here is an excellent article from the experts at Lallemand: https://www.wineland.co.za/new-tools-help-overcome-stuck-fermentations-wine/ New tools to help overcome stuck fermentations in wine by Anne-Ortiz Julien, Anthony Silvano, Didier Théodore, Françoise Raginel & Ann Dumont Twenty years ago, it was thought that stuck fermentations were mainly due to two things: nitrogen deficiency and the glucose/fructose ratio of the must. Recently, researchers have learned more about the topic, and we now know that other factors come into play. Stuck fermentation is where fermentation has ceased prematurely or the rate of fermentation is considered too low for practical purposes, leaving a higher residual sugar content than desired in the wines at the end of the fermentation (Figure 1) (Bisson, 1999; Henschke & Jiranek, 1993). Not only does it delay the completion of alcoholic fermentation (AF), but it can also lead to the formation of off-aromas. For winemakers, a residual sugar concentration of less than 2 g/ℓ is considered dry or completed (Bisson, 1999). FIGURE 1. Types of fermentation problems (adapted from UC Davis Wine Fermentation). What are the factors leading to stuck fermentation? Glucose/fructose ratio Saccharomyces cerevisiae is a glucophilic yeast, preferring glucose over fructose. During fermentation, glucose is consumed at a higher rate than fructose, and the proportion of fructose increases as fermentation progresses. This can lead to imbalances in the wines and, under the stressful conditions found at the end of fermentation, make it more difficult for wine yeast to utilise this non-preferred sugar. The preference for glucose over fructose is yeast dependent, and the discrepancy between glucose and fructose consumption is not a fixed parameter, but rather is dependent on the yeast’s genetic background and on external conditions (Berthels et al., 2004). Nitrogen deficiency Nitrogen is the most important yeast nutrient and has a significant impact on wine fermentation. It influences both fermentation kinetics and wine profile (Agenbach, 1977; Bezenger & Navarro, 1987). Grape musts contain nitrogen under different forms: proteins, peptides, ammonium ions (NH4+) and alpha amino acids. The yeast assimilable nitrogen (YAN) is composed of ammonium and a-amino acids, except proline which is not assimilable by yeast. The minimum quantity of YAN in must is 150 to 200 mg/ℓ, and lower levels are considered nitrogen deficient. When the must is deficient in nitrogen, it will limit yeast growth and fermentation speed (Bely et al., 1990; Julien et al., 2000). YAN content has the most influence on fermentation speed; it impacts yeast biomass at the beginning of fermentation, as well as sugar transport kinetics during fermentation (Figure 2). As soon as a must is nitrogen depleted at the end of the growth phase, there is a decrease in protein synthesis and sugar transport activity (Bely et al., 1994). If nitrogen deficiency is the only issue, and no other factors are lacking, the consequence is a slow or sluggish fermentation, but the yeast viability is maintained and AF can eventually be completed. FIGURE 2. Nitrogen depletion during the yeast growth phase (Sablayrolles, J.M., Sitevi Conference, 2015). Nutritional imbalances Recently, a study by Bruno Blondin’s research team (Duc et al., 2016) demonstrated that nitrogen starvation in AF does not trigger yeast mortality, and thus also does not trigger stuck fermentation. Indeed, in such conditions, yeast reacts with a system of stress responses to maintain its viability during all AF. On the contrary, lipid limitation does not induce any appropriate stress response by the yeast, leading to yeast cell death, and thus stuck fermentation. This yeast cell death is modulated by nitrogen level. In cases where there is a low lipid content and high nitrogen level, yeast mortality during AF is increased and can occur in the first stage of the fermentation (Tesniѐres et al., 2013). Studies have been conducted to identify other nutrient limitations that provoke yeast cell death. In cases where there are nutritional imbalances (e g, high nitrogen and starvation in oleic acid, panthotenate and nicotinic acid) high mortality have been observed (Figure 3). This very new data demonstrate the importance of a good nutritional balance (minerals, vitamins, sterols and organic nitrogen) not only to maintain yeast viability and avoid stuck fermentation, but also to limit the risk of off-flavours and deviations, such as H2S production. An imbalance between high YAN and low pantothenic acid levels in must significantly drives H2S production (Wang, 2003; Blondin, 2016). When choosing a nutrient, quality is as important as quantity, since only organic nutrients will provide those optimal growth factors, as well as organic nitrogen, while providing a better nutritional balance than with chemical DAP alone. FIGURE 3. Impact of various vitamins and sterols (in high YAN conditions) on yeast populations during AF. Vitamins & minerals Minerals, such as magnesium, zinc and potassium, are absolutely essential to the growth and metabolism of yeast (Walker, 1994). Minerals:
Vitamins are organic compounds essential to the optimum growth of yeasts cells and their capacity to survive under stressful conditions. Vitamin deficiencies can induce sudden changes in fermentation kinetics. The majority act as enzymatic cofactors. They can also intervene in energy transfers or in supporting membrane integrity. Biotin, for example, favours the production of esters and better cellular viability (Bohlscheid et al., 2007). When biotin is deficient, cellular growth is affected significantly (Figure 4). Pantothenic acid (Vitamin B5) helps prevent H2S production volatile acidity. Thiamine function of thiamine has been the subject of numerous studies, and thiamine deficiency can cause stuck fermentation. The concentration of thiamine has a large influence on fermentation kinetics; its presence increases the production of yeast biomass and fermentation speed (Bataillon et al., 1996). FIGURE 4. Glucose/fructose concentration during AF with Lalvin EC-1118™ in synthetic must (from Bohlscheid et al., 2007). Lack of oxygen and the role of sterols Oxygen plays an essential role in AF, fostering the development of an adequate yeast population and maintaining their vitality (Sablayrolles & Barre, 1986). Oxygen is required for the synthesis of survival factors, such as sterols and unsaturated fatty acids (David & Kirsop, 1973; Aries & Kirsop, 1978; Alexandre et al., 1994; Fornairon-Bonnefond et al., 2002), which are components of the yeast cell membrane. They play a key role in the membrane structure, helping maintain membrane fluidity, cell integrity and viability. During the yeast growth phase, each multiplication cycle dilutes the lipid content of the yeast cell. When lipids become insufficient, the yeast cell membrane does not function properly under limiting oxygen conditions. Sterols, located around the membrane proteins responsible for flux selectivity between the interior and the exterior of the cell, are no longer synthesised. During fermentation, as the ethanol level increases, yeast mortality increases. Without an adequate yeast sterol concentration, yeast cell wall permease activity suffers. When these proteins do not function adequately under increased ethanol concentration, there is an accumulation of H+ ions in the intracellular medium, and the yeast requires more energy to expel them outside the cell. This will rapidly lead to the acidification of the cell (drop of intracellular pH), resulting in cell death and stuck fermentation. So the more sterols are synthesised in the membrane, the better the resistance to ethanol. It is important to rehydrate the yeast cells with a yeast protector, such as the Natstep® range of products that will supply sterols to the yeast membrane (Soubeyrand et al., 2005). FIGURE 5. Yeast cell membrane and some of its constituents during AF. The sterols maintain membrane fluidity, resulting in a clean AF. Improper yeast rehydration and handling Proper yeast rehydration is a key step to successful AF as it is a crucial phase for the survival and efficiency of the wine yeast. If the yeast is not properly rehydrated, more than half of the yeast population can die. It is very important to follow manufacturer’s instructions, particularly with respect to both the rehydration medium and temperature. During yeast rehydration, the active dry yeast will absorb water and recover its original form. The organs inside the cell continue rehydrating and a part of them disperses in the rehydration water. This loss can represent between 20 – 30% of the yeast’s dry weight and can result in a micronutrient deficit. Rehydrating in wine must, at the wrong temperature and the recommended dosage, will all influence wine yeast performance. In order to initiate fermentation, there must be a minimum of 106 cells/mℓ. When using a 25 g/hℓ inoculum of active dry yeast, there will be approximately 5 x 106 cells/mℓ, which is usually sufficient for a regular AF, a good implantation in the must, and ultimately, no sluggish or stuck fermentation is rehydrated properly. For example, in Figure 6, the length of AF is shortened considerably when the dosage is at 20 g/hℓ (internal results). FIGURE 6. Impact of inoculation rate on AF. Lack of temperature management Fermentation temperature is important based not only on the type of wine to be fermented (red, white or rosé), but also the yeast used. Different yeasts, under different conditions, will be more sensitive to cooler or warmer temperatures during AF. Each selected yeast is characterised with a range of optimal AF performance, which must be carefully respected to avoid stuck fermentation. AF will be affected by extreme temperatures (either too low or too high), since it has been shown that ethanol toxicity increases at extreme temperatures. The yeast cell membrane is weakened, which eventually leads to cell death. Inhibitory metabolites Grape must composition may have inhibiting toxic compounds that affect yeast viability and fermentative activity and that are responsible for sluggish or stuck AF. Inhibiting toxic compounds, such as short and medium chain fatty acids (SMCFA), have been widely noted for their inhibition of AF. Pesticide residues (fungicide, herbicide and insecticide) can also seriously affect yeast viability and compromise the end of fermentation. Recent studies have also shown that they can negatively impact the production of aromas (namely esters) and fruit character of wine (Noguerol-Pato et al., 2014). How to cure a sluggish or stuck fermentation? Yeast cells have the extraordinary capacity to adapt to different stress factors in a difficult environment of the grape must (high sugar, low pH, gradual ethanol increase, nutrient depletion, competition with other microorganisms, etc). However, difficult conditions can prevail, resulting in a fermentation that won’t finish, and actions must be taken to cure stuck fermentation where the density is not decreasing anymore, or very slowly, and some off-aromas might be creeping up. Quick actions are necessary to avoid microbial spoilage and the appearance of wine faults (H2S, Brett odours, VA, etc) and to get fermentation going again. A reliable protocol that can be applied quickly and efficiently can help tackle the different causes of AF problems. Cleaning the must Grape must may contain toxic compounds that inhibit fermentation, such as short and medium chain fatty acids (SMCFA) and pesticides. A new generation of yeast hull products with good absorption capacity and an affinity for these types of compounds is now available. Trials have shown that yeast hulls, such as RESKUE™, favour complete and steady fermentations thanks to the removal of SMCFA and pesticide residues (Figures 7 and 8). FIGURE 7. Lab-scale trial Chardonnay (France, 2012). Level of SMCFA at the end of AF after addition of different cell-walls (40 g/hℓ) at the 3/4 of the AF. FIGURE 8. Lab-scale trials, white (A) and rosé (B) wines contaminated with several pesticide residues (Spain, 2013). Pesticide removal (%) after addition of Reskue™ at 40 g/hℓ. Using the right yeast Many factors influence the course of AF, and choosing the right yeast is crucial, especially when the conditions are difficult. When fermentation stops mid-stream, the must generally contains much more fructose than glucose, the type of sugar that yeasts prefer. To overcome this glucose/fructose imbalance, it is important to choose a yeast that has a strong affinity for fructose, the predominant sugar in stuck fermentation, but also a yeast that is alcohol tolerant and responds well to nutrient supplementation. In trials carried out over the years, one yeast stood out (Figure 9) among all selected yeasts available: Uvaferm 43™, which is more capable of fermenting fructose and has a very high fermentation capacity and a high alcohol tolerance. FIGURE 9. Fructose affinity of different wine yeast in synthetic medium MS300 260Glc/Fru; 24ºC; 25 g/hℓ. Even with these properties, at Lallemand R&D we drew on our premium knowledge of yeast growth and metabolism to develop a production process that makes yeast cells more resistant to stress conditions caused by high alcohol content. Uvaferm 43™ was then optimised and pre-acclimated during multiplication with specific micronutrients and survival factor protection. Survival factors include specific sterols and polyunsaturated fatty acids that strengthen the yeast membrane. As a result, yeast cells are then more robust, with a lower mortality rate after inoculation, and require less time to acclimate to the must. This simplifies the process of restarting your fermentation with only a few steps using the new Uvaferm 43 Restart™. In trials done with Uvaferm 43 Restart™, it was shown that an average of one week was gained when restarting a stuck ferment, as illustrated in Figure 10 in a Primitivo from Puglia, Italy. FIGURE 10. Sugar consumption with two different yeasts to complete fermentation after restarting a stuck Primitivo wine from Italy. In terms of fructose consumption, we can see that in both white and red wines, Uvaferm 43 Restart™ is very efficient for overcoming stuck fermentations. FIGURE 11. Fructose consumption in white and red wines with two different yeast (ISVEA, Tuscany, Italy). The right nutrition Right at the beginning of fermentation, vitamins, minerals and available nitrogen are consumed very quickly. This can cause sluggish and stuck fermentation as indicated above. It is then a key step to add nutrients naturally rich in these elements, such as Fermaid O™, to feed the yeasts when restarting a stuck fermentation. Fermaid O™ or Nutrient Vit Nature™ (when added at 1/3 sugar depletion) supply critical nutrients to help the yeast avoid stressed conditions. It is one of the best nutrients available for wine yeast as it only contains organic nitrogen and all the essential elements for the yeast to complete AF. An improved protocol We now understand that when restarting a stuck fermentation, even with a clean must/wine, the proper yeast and the best nutrients, using an efficient protocol will secure the process. With the help of the experts at Inter-Rhône (France), a protocol was developed to make the process simple, fast and efficient. FIGURE 12. Conclusion Proper fermentation management begins when choosing the yeast to ferment the wine and how it will be prepared for AF. A yeast adapted to must conditions and wine style development or to showcase the terroir is crucial. It must be rehydrated properly with a protector, such as Go-Ferm Protect™ or Go-Ferm Protect Evolution™, to ensure its membrane is in optimal shape in the difficult wine environment. It must be properly fed with the right nutrients, such as Fermaid O™. Sometimes, carrying out all the right preparations still may not be enough to overcome extreme or unmanageable conditions. When an AF is sluggish or stuck, there are now efficient and reliable ways to solve this issue. Proper must detoxification with Reskue™ yeast hulls and use of the robust Uvaferm 43 Restart™ as part of an easy-to-use protocol are sure ways to ensure wine quality. References Agenbach W. A. A study of must nitrogen content in relation to incomplete fermentations, yeast production and fermentation activity. Proc. South African Soc. Enol. Vitic., Cape Town, South Africa. Stellenbosch SA, 66-87. (1977). Alexandre H., T.N. Long, M. Feuillat, and C. Charpentier. Contribution à l’étude des bourbes : influence sur la fermentiscibilité des moûts. Revue française d’oenologie, 146, 11-19. (1994). Aries V and B.H. Kirsop. Sterols biosynthesis by strains of Saccharomyces cerevisiae in presence and absence of dissolved oxygen. J. Inst. Brew. 84: 118-122. (1978). Bataillon M., A. Rico, J.M. Sablayrolles, J.M. Salmon and P. Barre. Early thiamine assimilation by yeasts under oenological conditions: impact on alcoholic fermentation kinetics. J Ferm. Bioeng. 82: 145-150. (1996). Bely M., J.M. Sablayrolles, and P. Barre. Description of alcoholic fermentation kinetics: its variability and significance. American Journal of Enology and Viticulture. 41 (4), 319-324. (1990). Bely M., J.M. Salmon, and P. Barre. Assimilable nitrogen additions and hexose transport activity during oenological fermentations. J. Inst. Brew. 100:279-282 (1994). Berthels NJ, R.R. R.R. Cordero Otero, F.F. Bauer, J.M. Thevelein, I.S. Pretorius (2004) Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res 4(7):683-9. Bisson, L. 1999. Stuck and Sluggish Fermentations. Am J Enol Vitic. 50: 107-119. Bezenger M., J.M. Navarro. Influence de l’azote sur la fermentation alcoolique en milieu modèle simulant les conditions de l’oenologie. Sciences des aliments, 7, 41-60. (1987). Bohlscheid J.C. 1, J.K. Fellman2, X.D. Wang3, D. Ansen4, C.G. Edwards. Bohlscheid J.C. 1, J.K. Fellman2, X.D. Wang3, D. Ansen4, C.G. Edwards The influence of nitrogen and biotin interactions on the performance of Saccharomyces in alcoholic fermentations. David, M. and B. Kirsop. 19973. A Correlation between Oxygen Requirements and the Products of Sterol Synthesis in Strains of Saccharomyces cerevisiae. Journal of General Microbiology, 77, 529-53 I. Duc, C., I. Sanchez, M. Pradal, J. Noble2, A. Ortiz-Julien,C. Tesnière and B. Blondin. Yeast Cell Death Triggered By Nutrient Starvation in Wine Alcoholic Fermentation. Oral conference, PYFF6: 6th Conference on Physiology of Yeasts and Filamentous Fungi. Abstract Book, 11-14, July 2016, Lisbon, Portugal. Fornairon-Bonnefond C., V. Desmaretz, E. Rosenfeld, J.M. Salmon. Oxygen addition and sterol synthesis in Saccharomyces cerevisiae during enological fermentation. J. Biosci. Bioeng., 93, 176-182. (2002). Henschke P and V Jiranek. Yeast metabolism of nitrogen compounds. Wine Microbiology and Biotechnology. Fleet G.H. (Eds) 77-165. (1993). Julien A, J.L. Roustan, L. Dulau, J.M. Sablayrolles. Comparison of nitrogen and oxygen demands of enological yeasts: Technological consequences. Am. J. Enol. Vitic. 51 (3). (2000). Noguerol-Pato Raquel, Thais Sieiro-Sampredro, Carmen González-Barreiro, Beatriz Cancho-Grande and Jesús Simal-Gándara. Effect on the Aroma Profile of Graciano and Tempranillo Red Wines of the Application of Two Antifungal Treatments Onto Vines Molecules 19 (8), 12173-12193. 2014. Sablayrolles J.M. and P. Barre. Evaluation des besoins en oxygène de fermentations alcooliques en conditions oenologiques stimulées. Sci. Alim. 6 : 373-383. (1986). Soubeyrand V., V. Luparia, P. Williams, T. Doco, A. Vernhet, A. Ortiz-Julien, J.M. Salmon. Improvement of the Fermenting Capacity of Active Dry Yeast (ADY) by Solubilized Sterols during Rehydration. J. Agric. Food Chem. 2005. Tesnière C.1, P. Delobel, M. Pradal, B. Blondin. 2013. Impact of Nutrient Imbalance on Wine Alcoholic Fermentations: Nitrogen Excess Enhances Yeast Cell Death in Lipid-Limited Must. PLoS One 8 (4), e61645. Walker GM. The role of magnesium in Biotechnology. Critic. Rev. Biotechnol. 14: 311-354. (1994). (Walker et al. 2016). Wang X.D., J.C. Bohlscheid and C.G. Edwards. 2003 Fermentative activity and production of volatile compounds by Saccharomyces grown in synthetic grape juice media deficient in assimilable nitrogen and/or pantothenic acid Journal of Applied Microbiology, 94, 349-359. I did a post-doctoral research project at Opus One during harvest 2016 & 2017. Here is a recent article written by Ted Rieger of Wine Business Monthly. Ted and I talked after he saw my poster about tracing oxidation reduction potential (ORP) at ASEV 2018 in Monterey.
Here is the link to the original article: https://www.winebusiness.com/news/?go=getArticle&dataid=202368 Tracking Oxidation Reduction Potential in Wine Fermentations Opus One Using Redox Sensors to Analyze Fermentation Dynamics by Ted Rieger August 21, 2018 Opus One Winery in Napa Valley conducted a two-year research project in conjunction with UC Davis (UCD) to measure and monitor oxidation reduction potential (ORP) during red wine fermentations that proved to be an effective parameter to analyze fermentation dynamics. ORP is an effective indicator of yeast metabolism in fermentation and when it drops below a certain level, the fermentation enters a “reductive danger zone” where unwanted hydrogen sulfide (H2S) formation can occur. As a result of the research project, Opus One is moving forward with a goal to install ORP sensors to monitor and control the redox potential in all tank fermentations. The study, “Tracking Redox Potential during Fermentation as an Enological Parameter and Indicator of Yeast Metabolism,” was presented as a research poster at the 2018 National Conference of the American Society for Enology and Viticulture (ASEV) in Monterey, California. Dr. Gordon Walker, a wine biochemist, microbiologist and industry consultant, was the researcher who worked at Opus One Winery for two harvests following completion of his Ph.D at the UCD Department of Viticulture & Enology. The study was done in cooperation with Opus One winemaker Michael Silacci, Opus One interns Arlene Mains and Chris de Vries, and UCD enology professor Dr. Roger Boulton. Walker said he was inspired to conduct the experiment by Boulton’s work and teachings in this area, who has long called ORP “a hidden variable in winemaking.” Providing background, Silacci explained: “We did this study after years of conversations with Roger Boulton that date back to when I took classes at UCD in the 1980s. With Gordon’s work, we now have a good idea what level the ORP can drop to during fermentation to potentially produce H2S, and we can then push the ORP to a safer level through oxygen addition with a timely pumpover.” Unwanted H2S levels that persist in wine are a concern as they can later bind with ethanol to produce mercaptans that are more difficult to remove, and can produce volatile sulfur aromas such as “onion,” “rubber” or “skunk.” Traditionally, wine is treated with copper additions to mitigate mercaptan issues, but as Silacci explained, “We don’t know what possible long-term impacts copper can have on wine during aging, so our goal is to eliminate the use of copper.” Monitoring and Controlling ORP Redox potential is a reflection of the chemical environment of a system. The oxidation reduction potential (ORP) is a quantifiable measurement of the tendency of a molecule or ion to gain or lose electrons, and a measurement of the redox state. ORP is a common measurement in biochemistry, environmental chemistry and water quality. In aqueous solutions, ORP can be determined by measuring the potential difference between an inert sensing electrode in contact with the solution and a stable reference electrode. Walker and the researchers collected data during the 2016 and 2017 crush seasons at Opus One with fermentations of Cabernet Sauvignon in 2-ton stainless steel tanks. The tank setup used a Thermo Fisher Orion Versa Star pH meter (more commonly used for lab analyses) and platinum ORP probes. The calibrated probes were inserted into screen/pipe assemblies with the solution sensing electrode placed in fermenting must/wine below the cap within the tank and wired to the pH meter. ORP is measured in millivolts (mV). At Opus One, Walker said he saw ORP range from 400 mV to as low as 50 mV. The ORP value of must starts high, about 300 to 400 mV, but will begin to drop quickly with the onset of yeast activity and continues to drop as the yeast enter exponential growth. Walker found that when the ORP drops below about 200 mV, the fermentation enters a “reductive danger zone” where the elemental sulfur can be reduced to H2S. By tracking ORP, Walker said, “I could predict accurately when reduction would occur.” The Opus One winemaking staff confirmed that Walker’s ORP data accurately predicted reduction just before staff could begin smelling sulfur odors. The trials also demonstrated that higher pH and temperatures are associated with faster fermentations, leading to lower ORP values and more H2S character. A separate study by Boulton, recently conducted at UCD with faculty colleagues David Killeen and Andre Knoesen of the UCD Department of Electrical and Computer Engineering, demonstrated the ability to monitor and control redox potential at a chosen value by adding air during red wine fermentations. This experiment showed the redox potential could be controlled at 215 mV, well above its natural level, throughout the fermentation. A research paper on this work, “Advanced Monitoring and Control of Redox Potential in Wine Fermentation,” was recently published in the American Journal of Enology and Viticulture. In the Opus One study, the lowest ORP values corresponded to the yeast reaching a biomass maximum and ceasing growth. Yeast strains differ in their tendency to produce H2S, as a function of nitrogen requirements and how their metabolism drives the drop in ORP during fermentation. Opus One has identified and isolated five different “native” yeast strains from its estate that are desired for fermentations. Silacci believes they give Opus wines “a deeper expression of place.” Silacci said, “Our goal for fermentation is to use 80 percent wild yeasts and 20 percent commercial yeast using PDM.” The winery hopes to collect more data to correlate ORP values with yeast strains and grape sources. Walker has sequenced the genomes of these yeast strains with the goal of understanding their origins and contributions to quality. For the 2018 crush, Opus One is installing Hamilton industrial process ORP sensor probes through ports in the pumpover hard lines on four stainless steel fermenters—on two 10-ton tanks and on two 20-ton tanks. These EasyFerm Plus ORP Arc 120 sensors, manufactured by Hamilton Company Inc. based in Reno, Nevada, come with accessories for wireless real-time data monitoring. The sensors are designed for demanding applications in pharmaceutical and chemical industries. Walker, in addition to his consulting work, now serves as a sales rep with Flotek, based in Walnut Creek, CA, that represents Hamilton sensors and other products for food, beverage and chemical process applications. Opus is working with Acrolon Technologies of Sonoma, CA, producer of TankNET controls, to integrate the ORP sensors into overall tank control, monitoring and data management, that includes temperature control, Brix measurements and automatic pumpover scheduling and control. Tank pumpovers can also be controlled by a portable tablet device used by cellar workers. Opus One employee Mains said, ”Timing of oxygen addition is important, and having real-time data allows us to determine the time points that are most critical.” Assuming successful results for the 2018 crush, Silacci said, “Our goal is to put these ORP probes and systems on all of our tanks for 2019.” Opus also purchased a Hamilton EasyFerm Plus ORP Arc 425 sensor, a longer probe (425 mm) that will be used to take ORP readings of wine aging in individual barrels by manually inserting the probe through the bunghole during topping operations. Little is known about ORP during barrel aging and it has not been studied extensively. Mains said, “The barrel monitoring is exploratory and will be more for fact finding at this point to see if the ORP values we measure correlate to certain conditions or change over time.” Walker summarized, “ORP is a powerful predictive parameter for fermentation, and winemakers should definitely consider tracking this “hidden variable” in their fermentations. Tracking ORP is a great way to help winemakers understand what is happening in wine fermentations beyond just Brix and temperature.” |
Archives
March 2020
Categories
All
|

 RSS Feed
RSS Feed